Scientists can now grow flexible crystals

YOU’RE ALREADY familiar with substances that are crystals – think rock salt, or quartz. Typically, these materials are hard, brittle and inelastic, and crack or shatter irreversibly when struck or bent.
Published this week, our new paper describes a new type of crystal: one that is flexible, and that can even be tied in a knot.
This new development means we can think about new applications for crystals beyond their existing uses, such as in mobile phones and computers.
It’s all in the structure
The properties of crystals are due to the way that atoms or molecules are arranged; crystals have infinitely repeating molecular components. As well as defining their physical characteristics, these also result in useful features that underpin a wide variety of modern technologies, such as semiconductors in electronics.
But these physical properties also limit the use of crystals in emerging technologies like flexible electronics and optical devices.
Our new paper shows we can grow elastic crystals about the width of a fishing line and up to five centimetres long. These crystals can be reversibly and repeatedly bent and stretched as much as common plastics like Nylon or polyethylene. They show no signs of breaking or cracking. Remarkably, they also keep the useful properties of crystals.
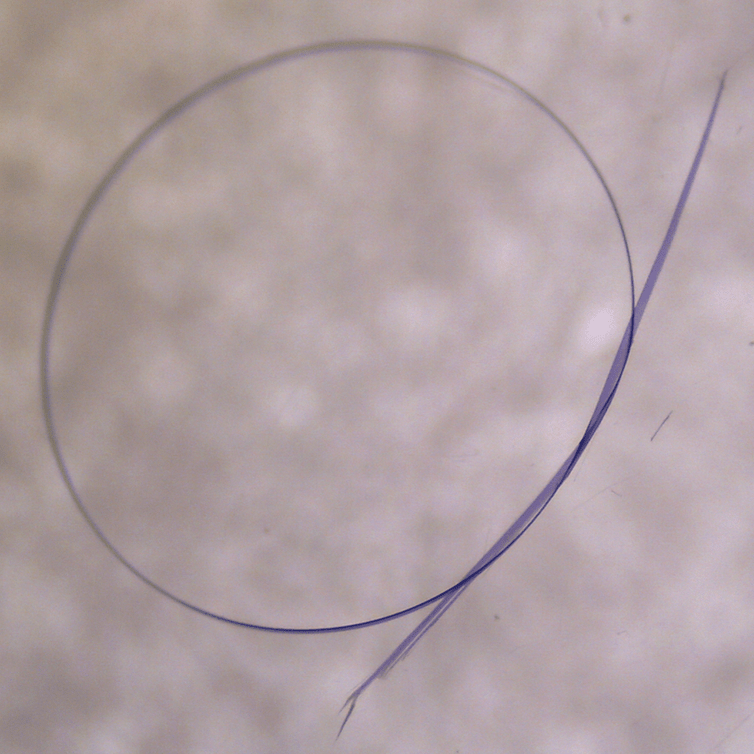
Yes, that’s a crystal. Tied in a knot. (Image Credit: Jack Clegg)
A new kind of crystal
The crystals that we have prepared fall outside the limits of what have traditionally been considered to be hard and soft matter.
The metal complex we used to make our crystal – copper (II) acetylacetonate – isn’t a new compound: it was first made in the late 1800s. But it’s how the molecules are arranged relative to the shape of the crystal that makes it different.
Using X-rays generated by the Australian Synchrotron, we were able to map changes in the structure of the crystals to the atomic level when they were bent. We found that the individual molecules reversibly rotate as they are bent, allowing both the compression and expansion that is required for elasticity.
Understanding the way that the molecules move when the crystals are bent will allow the development of many more examples. While we accidentally discovered the first example of these crystals, we have now made six more examples of flexible crystals containing related molecules.
Playing with Lego plus cooking
Making these crystals in the lab is a little bit like playing with Lego combined with cooking. We stick different molecular building blocks together in a flask, and after stirring and letting the liquid solvent evaporate we end up with beautiful flexible crystals.
Because we can control the chemistry of the building blocks on the atomic level we can also control the flexible properties of the crystal on the macro scale.
The method we have developed to measure the changes in the crystals during bending could also be used to explore flexibility in any other crystals. This is an exciting prospect given that there are millions of different types of crystals already known and many more yet to be discovered.
Bending the crystal changes its optical and magnetic properties, and our next step is to explore these optical and magnetic responses with a view to identifying applications in new technologies.
New materials, new technologies
The ability of crystals to bend flexibly has wide-ranging implications. For example, the loss of symmetry when a crystal is bent or twisted means it is no longer strictly a crystal by traditional definitions.
Flexible crystals like these will lead to new hybrid and new smart materials that respond to changes in their environment like temperature, pressure or the application of force for emerging technologies including components of aeroplanes and space craft or parts of sensors and electronic devices.
![]() The research is a joint study between researchers from Queensland University of Technology and The University of Queensland.
The research is a joint study between researchers from Queensland University of Technology and The University of Queensland.
Jack K. Clegg is an A/Prof. of Inorganic Chemistry at the The University of Queensland and John McMurtrie is an Associate Professor of Inorganic Chemistry at the Queensland University of Technology
This article was originally published on The Conversation. Read the original article.
READ MORE:




